Non Transition Elements in D Block
Scandium has the electronic structure Ar 3d 1 4s 2. Valence shell electronic configuration is n-1d1-10 ns1-2.
D Block Elements Iit Jee Study Material With Properties And Examples
Therefore no of elements present in it is 402since La Ac arent included38 there are 1515 30 elements present in F-orbital.
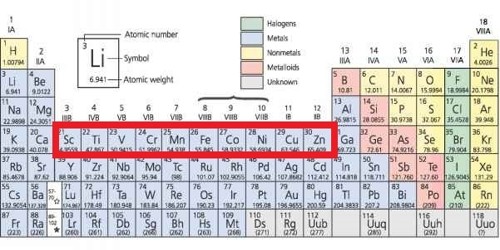
. II- B Zn Cd and Hg and III- A Sc Y La and Ac are non-typical transition elements while the remaining are typical transition elements. Cr 3d 5 4s 1 Nb 4d 4 5s 1 Pb 4d 10 5s 0 Ag 4d 10 5s 1 Cu 3d 10 4s 1 Mo 4d 5 5s 1 Ru 4d 7 5s 1 Pt 5d 0 6s 1 Au 5d 10 6s 1. When it forms ions it always loses the 3 outer electrons and ends up with an argon structure.
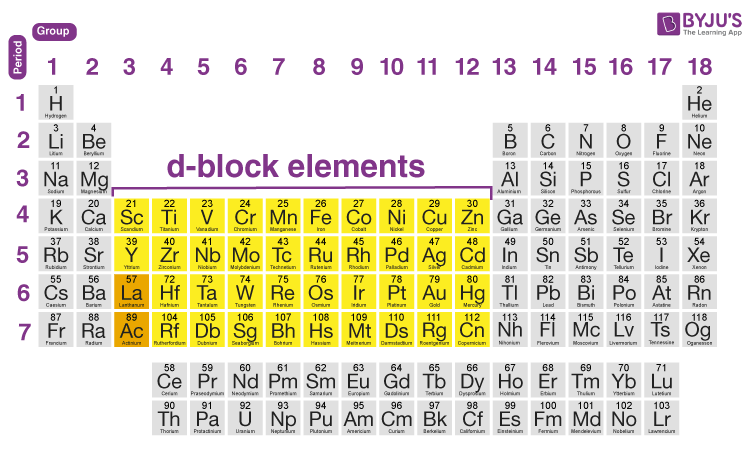
D-block elements are also known as the transition metals. Elements in the 12 columns of the d block such as Zn Cd and Hg have entirely filled d-orbitals and are hence not considered transition elements. The transition elements that are the d-block elements lie in between these metallic.
Some exceptional electronic configuration of transition element are. On the basis of this definition scandium and zinc do. Transition elements have partially filled d orbitals such as Fe CO Ti and non-typical transition elements have fully filled d orbitals such as Zn Cd and Hg since their inner or penultimate d-orbitals are.
The elements like Zn Cd and Hg are exceptions and known as non-transition elements as their orbitals are completely filled in their ground state as well as in their general oxidation state. Transition and d-block elements 1 A transition element is one which forms one or more stable ions with incompletely filled orbitals. The three series of transition metals are known 3d series 4d series and 5d series.
Zn Cd Hg are called non typical transition element. They also do not show the characteristics of transition element. On the basis of this definition scandium and zinc do not count as transition metals - even though they are members of the d block.
The Sc 3 ion has no d electrons and so does not meet the definition. Here there are 10 GROUPS 4 PERIODS in D-block. 2 A d-block element is one which has electrons filling the d-orbitals.
Some examples of elements which are in d-block but not a transition metal Scandium has the electronic structure ceAr 3d1 4s2. Scandium has the electronic structure Ar 3d 1 4s 2. D-Block Elements These elements are called transition elements.
Metals are in the s block nonmetals are in the p block but metals nonmetals and metalloids are in the d block. The correct statements are. Iv All members of d-block series are stable elements and found in nature.
Also to know is what is not a transition element. Transitions elements are the elements which form stable ions having incompletely filled d -orbitals. When it forms ions it always loses the 3 outer electrons and ends up with an argon structure.
It is clear that d-block elements have d-electrons in the d-sub shell. Transition Elements get their name from the fact that they are placed between s and p block elements. This block contains the elements of groups 3 to 12 of the periodic table.
3 From the above definition scandium and zinc are not counted as transition elements although they are d-block elements. A transition metal is one which forms one or more stable ions which have incompletely filled d orbitals. I There are four d-block series.
This happens as each additional electron enters the penultimate 3d shell. For example Zinc and Scandium are d-block elements. We can conclude that A transition metal is one which forms one or more stable ions which have incompletely filled d orbitals.
But those d-block elements or their ions which do not have incompletely filled d-orbitals are not transition elements eg Zn Cd and Hg are d-block elements but not transition metals. All transition elements exhibit similar properties because of the identical electronic configuration of their peripheral shell. The d block elements will have paired electrons in the n-1d shell and hence show some diamagnetism.
When it forms ions it always loses the 3 outer electrons and ends up with an argon structure. The d-block elements are found in groups 3 4 5 6 7 8 9 10 11 and 12 of the periodic table. But not transition elements.
For example- Sc Fe Os etc. For example Scandium has electronic configuration Ar 4s 2 3d 1 is a transition element but Zinc has electronic configuration Ar 4s 2 3d 10. Transition elements are present in the d block and their d subshells are partly filled in the ground state.
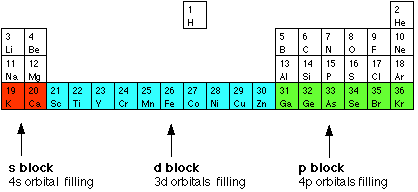
What is d block elements. 217 The d- and f- Block Elements The electronic configurations of outer orbitals of Zn Cd Hg and Cn are represented by the general formula n-1d10ns2. In d-block elements a valence electron enters in d-orbital.
Transition elements Chapter 24 The transition elements are found in the d block of the Periodic Table between Groups 2 and 13 A transition element is a d-block element that forms one or more stable ions with an incomplete d subshell We do not define Sc and Zn as transition elements. Ii Total d-block elements are 40. They also possess ferromagnetism which is mainly exhibited by the elements like cobalt and nickel along with iron.
The s block elements are metals while the p block elements are non-metals. Iii Last d-block series starts with element actinium. Scandium has the electronic structure Ar 3d 1 4s 2.
Many metals contain the paramagnetic property as they have more no of unpaired electrons. The periodic table is divided into s p d and f block elements. On the basis of this definition scandium and zinc do not count as transition metals even though they are members of the d block.
The orbitals in these elements are completely filled in the ground state as well as in their common oxidation states. On the basis of the definition outlined above scandium and zinc dont count as transition metals - even though they are members of the d block. Answer 1 of 2.
On the basis of this definition scandium and zinc do not count as transition metals - even though they are members of the d block. In this video we will discuss the inorganic chemistry long question d block elementin this video heading are.

All Transition Elements Are D Block Elements But D Block Elements Are Not Transition Qs Study

Electronic Configuration And General Properties Of D Block Elements Or Transition Elements Online Science Notes
Comments
Post a Comment